Note-Please Attempt problem from your own first.Otherwise click on the link question to get the solution.
Then the only nonnegative solution u of (4.2.2) is the trivial solution. Part A) of Lemma 4.2.2 was first proved in 36 for p, q 1, and later generalized in 49 to the full range.Part B) was obtained in 49 for solutions having algebraic growth at infinity and generalized in 39 for all nonnegative solutions.Finally, Part C) is due to 17.We also have the following Liouville theorem for. NCERT book for class 9 Science subject is available here. Students may read and download each chapter in PDF format. Also check precise NCERT Solutions for class 9 Science. Balbharati solutions for English - My English Coursebook 10th Standard SSC Maharashtra State Board chapter 3 (Let us March!) include all questions with solution and detail explanation. This will clear students doubts about any question and improve application skills while preparing for board exams. The detailed, step-by-step solutions will help you understand the concepts better and clear your. Solution: Given that 3 cot A = 4. Let us assume that Δ ABC is a right triangle, right angled at vertex B. Cot A = Side adjacent to ∠A Side opposite to ∠A ⇒ AB BC = 4 3 Let AB and BC be 4R and 3R respectively, where R is a positive number.
Chapter 5 Problem [D][a]
Chapter 5 Problem [D][b]
Chapter 5 solutions Problem[D][d]
Chapter 5 solutions Problem[D][e]
Chapter 5 Solutions Problem[D][c]
Chapter 5 Problem[F][a]
main( ), finds the product of these two and returns the product which is printed through main( ).
Chapter 5 Problem[F][b]
Chapter 5 Problem[F][c]
Chapter 5 Problem [J][a]

Chapter 5 Problem [J][b]
Chapter 5 Problem[J][c]
Chapter 5 solutions problem [J][e]
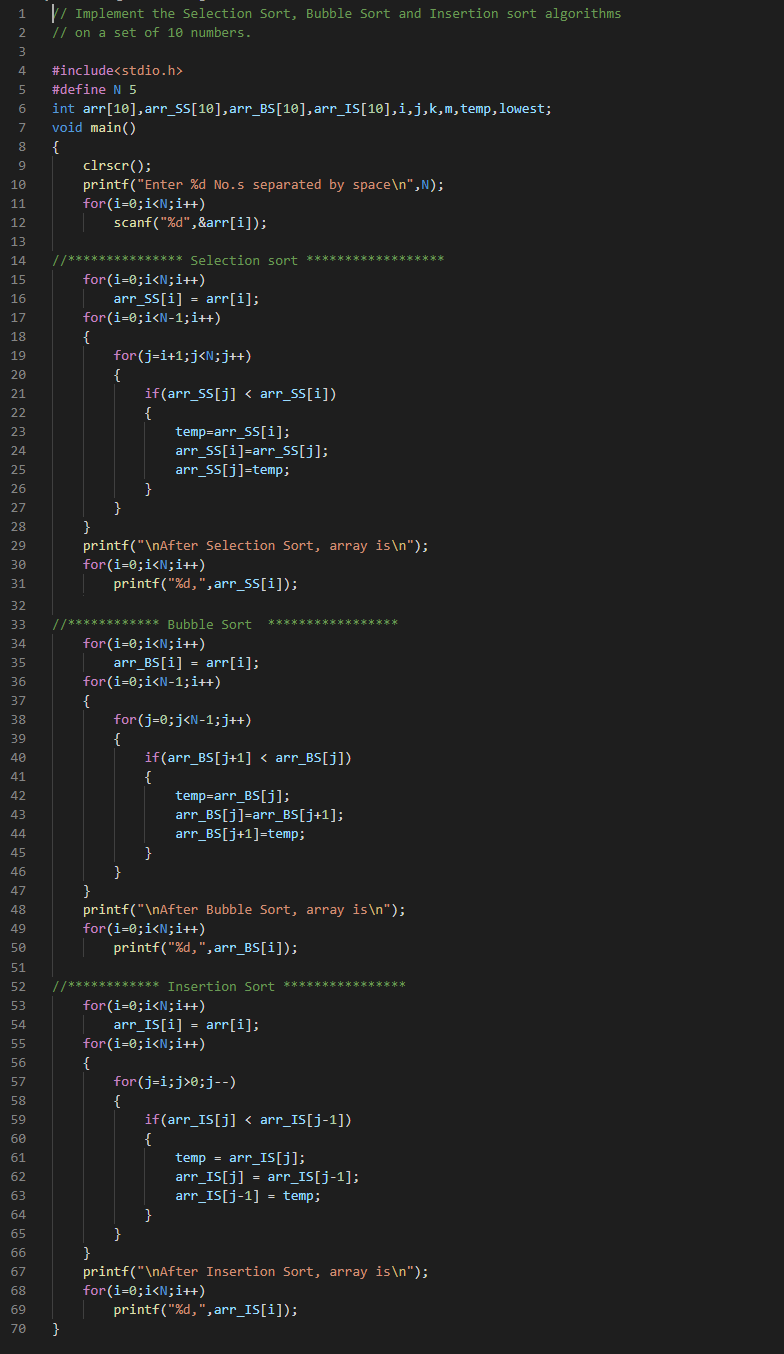
Chapter 5 Problem [J][f]
Chapter 5 Problem [J][g]
chapter 5 Problem [J][k]
Chapter 5 problem [J][j]
Chapter Problem [D]
Chapter 6 Problem [A][c]
chapter 6 solutions problem [A][d]
main( )
{
int x, y, s = 2 ;
s *= 3 ;
y = f ( s ) ;
x = g ( s ) ;
printf ( 'n%d %d %d', s, y, x ) ;
}
int t = 8 ;
f ( int a )
{
a += -5 ;
t -= 4 ;
return ( a + t ) ;
}
g ( int a )
{
a = 1 ;
t += a ;
return ( a + t ) ;
}

Living Science Solutions for Class 6 Science Chapter 6 Changes Around Us are provided here with simple step-by-step explanations. These solutions for Changes Around Us are extremely popular among Class 6 students for Science Changes Around Us Solutions come handy for quickly completing your homework and preparing for exams. All questions and answers from the Living Science Book of Class 6 Science Chapter 6 are provided here for you for free. You will also love the ad-free experience on Meritnation’s Living Science Solutions. All Living Science Solutions for class Class 6 Science are prepared by experts and are 100% accurate.
Page No 65:
Question 1:
In a physical change
(a) the molecules of the substances do not change.
(b) the molecules of the substances change.
(c) atoms forming the molecules undergo changes.
(d) new substances are formed.
Answer:
(a) The molecules of the substances do not change.
Physical changes are reversible, the molecules of the substance remain exactly the same before and after the physical change.
Page No 65:
Question 2:
Growth in plants and animals is
(a) a physical change.
(b) a chemical change.
(c) neither a physical change nor a chemical change since it is a life process.
(d) either a physical change or a chemical change depending on how much the growth is.
Answer:
(b) a chemical change
Growth occurs due to the intake of food. The substances present in food are used to nourish the cells and allow them to grow/divide. Hence, growth is a chemical change in which the molecules present in the food undergo changes to form molecules that enhance growth.
Page No 65:
Question 3:
If two substances are brought in contact, it will result in
(a) a physical change only.
(b) a chemical change only.
(c) either a physical change or a chemical change, depending on the conditions.
(d) a chemical change if they are heated together otherwise, a physical change.
Answer:
(c) either a physical change or a chemical change, depending on the conditions.
When two substances are mixed together, it can result either in a physical or chemical change. This depends on the substances that are mixed and also on the conditions.
Page No 65:
Question 4:
Chemical changes are
(a) always irreversible.
(b) mostly irreversible.
(c) always reversible.
(d) mostly reversible.
Answer:
(a) always irreversible.
Chemical reactions always result in forming new substances with different molecules. It is not easy to get back the original substances. Hence, the chemical changes are permanent and irreversible.
Page No 65:
Question 5:
Which of these is an irreversible change?
(a) melting of ice
(b) melting of wax
(c) boiling of water
(d) burning of wax
Answer:
(d) burning of wax.
In the process of burning of wax, it is neither the solid wax nor the liquid wax that is burnt. Wax vapours are burnt giving off heat and light. Once burned, it is not possible to get back the wax. Burning of wax is a chemical process and hence, is irreversible.
Page No 65:
Question 6:
Which of these is a reversible change?
(a) milk turning into curd
(b) burning of wax
(c) melting of wax
(d) rusting of iron
Answer:
(c) melting of wax.
The melted wax gets solidified after cooling. The melting of wax, therefore, is a reversible change.
Page No 65:
Question 7:
Which of the following is a physical and reversible change?
(a) heating of iron and sulphur to form iron sulphide
(b) melting of ice to form water
(c) weathering of rocks
(d) cooking of rice
Answer:
(b) melting of ice to form water
The melting of ice to give water is a physical and reversible change. The water can be frozen to again form ice.
Page No 65:
Question 8:
Which of these is a physical and irreversible change?
(a) burning of a paper
(b) evaporation of water
(c) breaking of a glass tumbler
(d) lighting of an electric bulb
Answer:
(c) breaking of a glass tumbler
The glass tumbler or its broken pieces are made of glass and there are no new molecules created, so its is a physical change. However, you cannot get back the glass from the broken pieces so, the change is irreversible.
Page No 65:
Question 1:
Every change has a cause. True or false?
Answer:
True.
A substance continues to remain either in its physical state or chemical state until and unless it is acted by an external factor like heat, motion, wind etc. So, every change has a cause.
Page No 65:
Question 2:
Every change can be classified as either physical or chemical. True or false?
Answer:
True.
Sometimes a change in a substance may result in the formation of new substance and sometimes may not. Depending on this, every change can be classified as either physical or chemical.
Page No 65:
Question 3:
Is dissolving sugar in water a physical change or a chemical change?
Answer:
Sugar dissolving in water is a physical change. The sugar can be obtained back by evaporation of water and the water by condensation of water vapour.
Page No 66:
Question 1:
Give one example for each of the following types of changes:
(a) physical changes.
(b) chemical changes
(c) reversible changes
(d) irreversible changes
Answer:
(a) Physical changes:
Example: Change in the size of the pencil or eraser with continuous use.
(b) Chemical changes:
Example: Burning of wood.
(c) Reversible changes:
Example: Salt dissolving in water.
(d) Irreversible changes:
Example: Curdling of milk.
Page No 66:
Question 2:
A potter shapes pots out of clay. He then bakes the pots in an oven. Identify the reversible and irreversible changes taking plance in the process.
Answer:
A potter shapes pots out of clay. This is a physical change which can be easily reversed. During this change, only the shape of the substance changes, without any change in the texture of the clay.
The potter then bakes the pots in an oven that makes the clay to becomes hard and brittle. Thus, there is a change in the internal structure of clay which makes soft clay hard and brittle. This is a chemical change which is irreversible.
Page No 66:
Question 3:
Breaking of a glass tumbler and burning of paper are changes in which we cannot get back the original substances. What is the difference between these two changes?
Answer:
| Breaking of glass tumbler | Burning of paper |
| It results in the change of the shape of the tumbler. | It results in the formation of smoke and water vapour. |
| The molecules that make up the glass remain the same. | The molecules of paper are converted to the molecules of ash. |
| This is a physical change, as no new substances are formed. | This is a chemical change, as paper is converted to ash. |
Page No 66:
Question 4:
What kind of change is cooking of food? Why?
Answer:
Cooking food is an irreversible, chemical change. During cooking, the molecules that are present in food changes to form new substances. Also, cooked food cannot be reverted to the raw state.
Page No 66:
Question 5:
Do you agree with the statement 'all physical changes are reversible'? If not give the correct statement.
Answer:
No, there are some physical changes which are irreversible. For example, tearing of a paper. When a paper is torn, the size and the shape of the paper changes, but the molecules of the paper remains the same. Since, no new molecules are formed, tearing paper is a physical change. At the same time, we cannot get back the sheet of paper from the pieces. Thus, the physical change is irreversible.
Page No 66:
Question 6:
Growth is increase in size. Is it a physical or a chemical change? Give reasons.
Answer:
Growth results in increase in size due to the intake of food. The food consumed by a living organism, is absorbed by the body and is used to nourish the cells for growth. Therefore, this is a chemical change, which includes the formation of new substances during the process of growth.
Page No 66:
Question 1:
Explain the difference between physical and chemical changes, giving one example of each.
Answer:
| Physical change | Chemical change |
| No new substance is formed. | New substance is formed. |
| The molecules of the substances remain the same before and after the change | The new substances formed during the change exhibit different properties from that of the original substances. |
| Example: Heating of iron. When heated, a piece of iron expands, but it contracts to its original size when cooled. | Example: Curdling of milk. The molecules of milk are changed in the curdling process to give curds. |
Page No 66:
Question 2:
'One way changes are classified is reversible and irreversible'. Explain with examples.
Answer:
Chapter 3let Us C Solutions Inc
Consider the example, when salt is mixed with water. The salt dissolves in water forming a solution. The salt can be obtained back by the evaporation of water and water by condensation of the water vapour. Such changes which can be easily reversed are called reversible changes.
Consider the example of rusting of iron. When an iron is kept in humid air for some days, a brown substance (rust) deposits on it, which has very different properties from that of iron. There is no simple way to get back the iron from the rust. Such changes which cannot be reversed are called irreversible changes.
Page No 66:
Question 3:
What is a chemical reaction? Explain with an example.
Answer:
A chemical reaction is the process by which reactants react with each other to yield products. In chemical reactions, the reactant molecules are completely different from the molecules of the products formed.
Example: Take some washing soda and mix it with water in a bottle. After making sure that the washing soda is fully dissolved in the water, add a few drops of lemon juice to the contents of the bottle. Hold a lit match stick near the mouth of the bottle. You will see that the match is extinguished showing that the gas produced is carbon dioxide.
Page No 66:
Question 4:
Give one example to show that when substances are mixed, it may result in a chemical or a physical change depending on the conditions.
Answer:
When substances are mixed together, it can result either in a physical change or a chemical change.
Example: If iron and sulphur are mixed, no chemical change occurs. But when the mixture is heated, it glows after some time and a black substance is formed which is different from iron and sulphur. So, heating a mixture of iron and sulphur results in a chemical change.
Similarly, when sugar and water are mixed together, sugar dissolves completely in water. No new substances are formed during the change. The sugar and water can be obtained back from the solution. This is a physical change which can be reversed.
Page No 66:
Question 5:
When a candle burns, both physical and chemical changes occur. Explain.
Answer:
When a candle burns, wax in the candle melts and is then vaporised as it is drawn up the wick. Melting and vaporisation are physical changes. The wax vapours then burn at the wick to leave behind soot and water vapour, while emitting heat and light. The burning of wax vapours is a chemical change. Thus, wax undergoes both physical and chemical changes when a candle burns.
Page No 66:
Question 4:
Is change of state of physical change or a chemical change?
Answer:

The change of state of a substance does not involve the formation of new molecules. A change in temperature or pressure can reverse the change in state. Therefore, a change in state is a physical change.
Page No 66:
Question 5:
Most chemical changes cannot be easily reversed. Such changes are called ___________changes.
Answer:
Most chemical changes cannot be easily reversed. Such changes are called irreversiblechanges.
Page No 66:
Question 6:
Is changing of milk into curd a reversible change?
Answer:
No, changing of milk is not a reversible change. When the milk changes into curd, the molecules of the milk undergo change resulting in the formation of curd.
Page No 66:
Question 7:
Can you get burnt paper back to its original form?
Answer:
When a paper is burnt, the molecules of paper undergo change forming new substances like smoke and water vapour. The change here is irreversible. So, a burnt piece of paper cannot be brought back to its original form.
Page No 66:
Question 8:
Substances expand on heating. Is that a physical change or a chemical change?
Answer:
Substances which expands on heating, regain their original shape on cooling without forming any new substances. This change is a physical change.
Page No 66:
Question 1:
Tearing of paper is irreversible. It is, therefore, a chemical change. Do you agree? Give reasons.
Answer:
The tearing of paper is an irreversible change as we cannot get back the original sheet of paper from the pieces. However, there are no new substances created by tearing paper. The pieces of paper obtained have the same molecules as the original sheet of paper. Thus, tearing of a paper sheet is a not a chemical change, but it is a physical change.
Page No 66:
Question 2:
A potato remains a potato even after it is cooked. Therefore, cooking is a physical change. Do you agree? Give reasons.
Answer:
When a potato is cooked, heating the potato cause changes in the molecules of the potato. Therefore, upon cooking, the potato does not remain the same at a molecular level. Thus, cooking is a chemical change, not a physical change.
Page No 66:
Question 3:
Chapter 3let Us C Solutions Llc
When sugar dissolves in water, it disappears. That means it must have changed into a new substance. Therefore, dissolving is a chemical change. Do you agree? Give reasons.
Answer:
When sugar dissolves in water to form a sugar solution, the sugar disappears. However, the molecules of sugar remain intact and are distributed uniformly in the water. Also, the water molecules are not changed when sugar is dissolved in water to form a sugar solution.
We can easily get back the sugar by evaporation of water from the sugar solution. The evaporated water can then be condensed to recover the water also. Hence, it is a physical change and not a chemical change.
View NCERT Solutions for all chapters of Class 6
